InterVenn’s biomarker discovery platform, GlycoVision™, is the only commercially available quantitative glycoproteomic biomarker discovery platform capable of accessing a historically hidden, information-rich layer of biology.
Contact us to partnerHistorically, the complexity of data generated using mass spectrometry had made it virtually impossible to study the glycoproteome at scale. Using artificial intelligence and machine learning to accelerate data processing, GlycoVision has overcome this barrier and is generating clinically relevant information that is missing from the existing -omics landscape.
GlycoVision is the only commercially available glycoproteomic platform that enables the generation of new disease insights and the discovery of a robust pipeline of biomarkers for early disease detection, response prediction, disease prognosis, and therapeutic monitoring. Together we can discover a spectrum of novel, high-performing biomarkers to revolutionize our understanding of disease pathways and therapeutic response.
GlycoVision detects approximately 700 of the most highly abundant glycoisoforms found in blood with well-established disease involvement. The platform generates fast and reproducible results to help select patients for clinical trials, monitor efficacy of therapy, and enable new drug target identification. Our technology does not depend on biomarkers that are produced by a tumor; instead, it relies on an amplification orchestrated by the highly sensitive host immune system, enabling detection at the earliest stages of disease.
We offer three powerful GlycoVision panels to meet your clinical research needs:
GlycoVision Discovery: Our flagship biomarker discovery service, offering unparalleled access and insight into the glycoproteome, recently updated to offer class-leading coverage, scalability, and robustness. Features dynamic insight into post-translational modifications of immunoglobulins, complement factors, & acute phase reactants. Coverage includes 628 glycopeptides.
GlycoVision Focus: our GlycoVision panel tuned to the core set of glycopeptides that are most strongly linked to disease involvement across a wide range of diagnostic applications. Coverage includes 442 glycopeptides.
GlycoVision Immune: our targeted panel of immune-specific glycopeptides, immunoglobulins and complement factors, curated both for adaptive and innate host immune response. Coverage includes 194 glycopeptides.

Biomarker Discovery and Validation
Choose an unbiased or targeted approach.

Pathway Analysis
Discover new insights into biomarker-related pathways.

Patient Stratification
Stratify patients with response prediction, prognosis, and monitoring.

Clinical Trial Design Support
Insights into clinical trial design, patient selection, and other clinical endpoints.
InterVenn has developed a complete glycoproteomic solution: our scientists work hand in hand with your team all the way from study design to analysis and data interpretation.
less than
100µL
required per sample

Accessioning and Extraction


Sample Preparation and Enzymatic Digestion


Separation and Identification by Liquid Chromatography-Mass Spectrometry (LC-MS)

Proprietary AI & Machine Learning


Reporting
FRONTIERS IN IMMUNOLOGY
Plasma glycoproteomic biomarkers identify metastatic melanoma patients with reduced clinical benefit from immune checkpoint inhibitor therapy
Front. Immunol., 13 June 2023 | Sec. Cancer Immunity and Immunotherapy | Volume 14 - 2023
Immune checkpoint inhibitors are effective treatments for advanced or metastatic melanoma, but not all patients show durable response.
Compared to prior studies, like the one seen in Figure 1, we improved separation and ability to select patients who will benefit from IO.
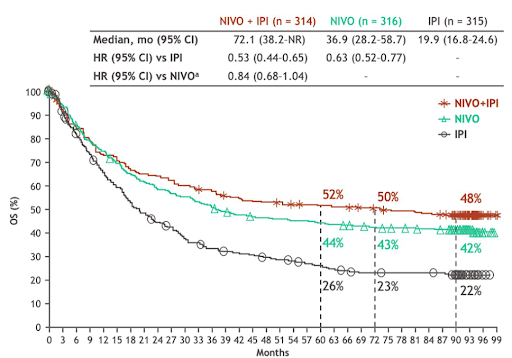
Figure 1. Journal of Clinical Oncology 2022 40:16_suppl, 9522-9522
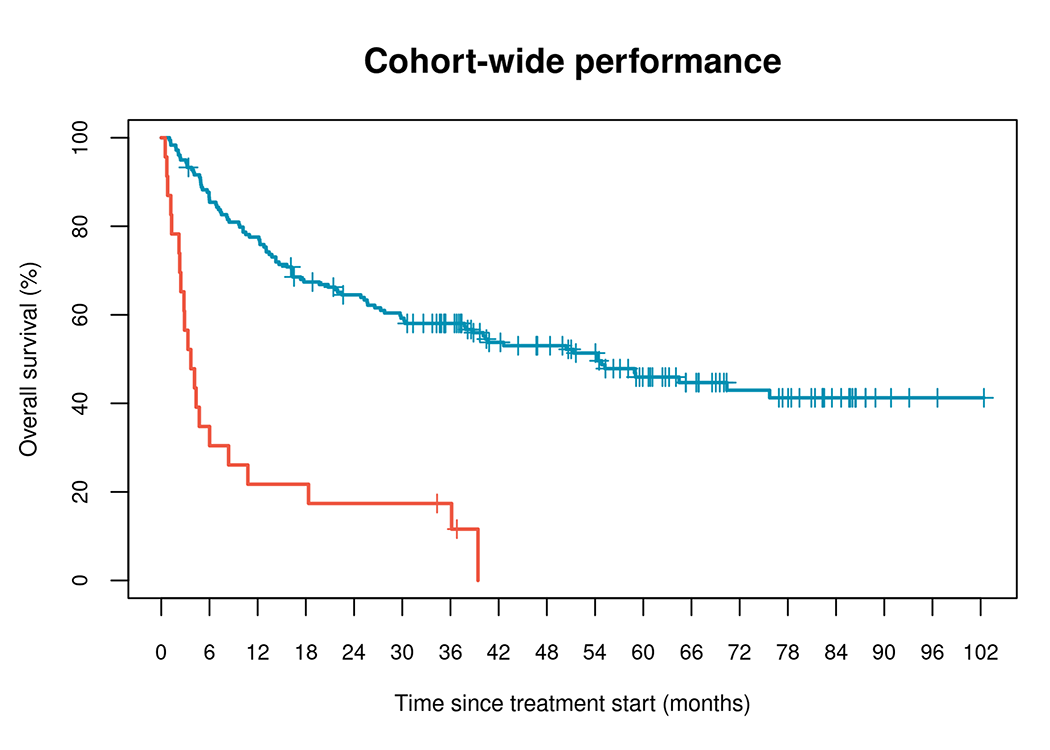
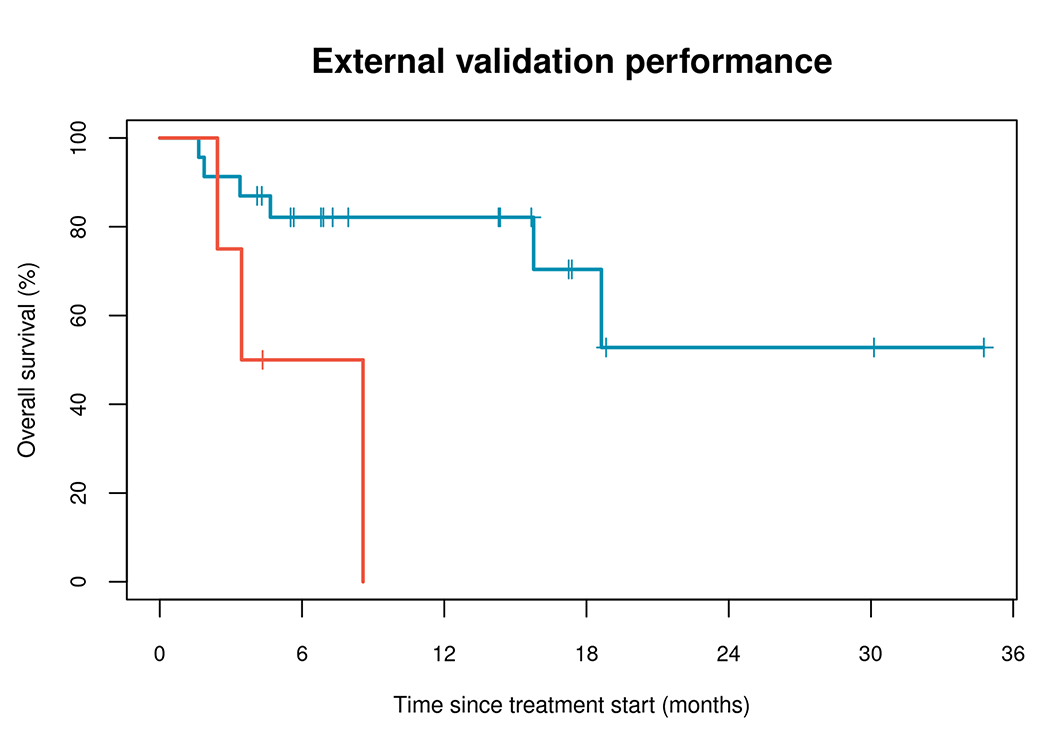
– Likely to Benefit
– Unlikely to Benefit
| Classifier prediction | Events/N | Median OS (Months) | HR (95% CI) | P-value |
|---|---|---|---|---|
| Discovery cohort (n=202) | ||||
| Likely to benefit | 92/179 | 54.3 (37.9, NR) | Reference | |
| Unlikely to benefit | 21/23 | 3.7 (2.4, 10.8) | 5.1 (3.1, 8.4) | 7.6×10-11 |
| Independent validation cohort (n=27) | ||||
| Likely to benefit | 6/23 | NR (15.8, NR) | Reference | |
| Unlikely to benefit | 3/4 | 6.0 (2.4, NR) | 5.6 (1.2, 25.5) | 0.027 |
We have identified biomarkers that can predict which patient populations are likely or unlikely to benefit from anti-PD-1 monotherapy or anti-CTLA-4/PD-1 combination therapy.
The initial model was developed using a discovery cohort of 202 patients recruited as part of studies conducted at the Massachusetts General Hospital (MGH). These patients had metastatic melanoma treated with first or second-line anti-PD-1 monotherapy or anti-PD-1/anti-CTLA-4 combination therapy.
The model was further tested with an independent validation cohort to confirm the performance characteristics of the classifier. This cohort of 27 patients was recruited as part of studies conducted at Royal Adelaide Hospital. The cohort included patients with metastatic melanoma treated with first or second-line anti-PD-1 monotherapy (pembrolizumab or nivolumab) or anti-PD-1/anti-CTLA-4 combination therapy (nivolumab/ipilimumab).
Early Detection of Advanced Adenomas and Colorectal Carcinoma by Serum Glycoproteome Profiling
Gastroenterology
Read PaperVariable PD-1 glycosylation modulates the activity of immune checkpoint inhibitors
Life Science Alliance
Read PaperNovel plasma glycoprotein biomarkers predict progression-free survival in surgically resected clear cell renal cell carcinoma
Urologic Oncology: Seminars and Original Investigations
Read Paper
Glycoproteomics provides a unique perspective by defining the roles of glycosylation in regulating health and disease. It complements other -omics by contributing detailed information about protein modifications that critically influence protein function and stability, offering insights into disease mechanisms that are not apparent through other -omics analyses alone.
The integration of data from various -omics fields, including glycoproteomics, is a powerful approach for obtaining a comprehensive understanding of biological systems.
By clicking submit, you confirm that you are a U.S. resident and you agree to receive information about InterVenn, including information about any of its current or future products or product candidates or disease state information, and you acknowledge that you have read and agree to the Terms of Use and Privacy Policy. All personal information will be kept confidential and will not be shared with any parties other than InterVenn, its affiliates and their designated agents.
View our privacy policy.